The SARS-CoV-2 virus is the novel coronavirus responsible for the devastating scenario that we, the citizens of the world, currently find ourselves in.
Without going into too much detail, and as most people know by now, the SARS-CoV-2 virus first appeared in Wuhan, China, in the last days of 2019. It rampaged through the world and continues to spread seemingly unabated. Statistics, as compiled by John Hopkins University, show that, to date, over 27 million people have been infected by the virus, with 889 364 fatalities.
As an aside, this is why the disease caused by the coronavirus is known as COVID-19. The “19” stands for the fact that the virus was first seen in 2019. And the “COVID” is part of the term coined by the World Health Organization that refers to the coronavirus responsible for the disease.
Because this coronavirus is new; ergo has never been seen before, scientists and medical professionals knew very little about its behavior. Time as passed, and there are many research studies into the virus’s behavior, including its genomes, how it mutates, how it is transmitted, and how to treat the associated disease.
The second aspect of the SARS-CoV-2 virus is that many of the world’s research scientists are dealing with is developing a vaccine. It is generally believed that the only way to control and prevent the virus’s spread is by creating an effective vaccine.
Enter COVID-19 convalescent plasma.
While all the other aspects of learning about the coronavirus’s behavior are necessary, let’s focus on a treatment regime looks promising.
Statnews.com reports that studies, while not yet peer-reviewed, demonstrate that convalescent plasma treatment is safe.
The Mayo Clinic released data in June 2020 that showed that “plasma treatment was safe following transfusion in a group of 20,000 patients, which included substantial enrollment from Black and Latino patients.”
Consequently, on 23 August 2020, the FDA announced emergency authorization for the use of convalescent plasma to treat COVID-19.
These statements have spawned several questions that are valid and deserve considered answers.
Therefore, by way of providing answers to these questions, let’s consider them one at a time.
What is convalescent plasma and how does it work?
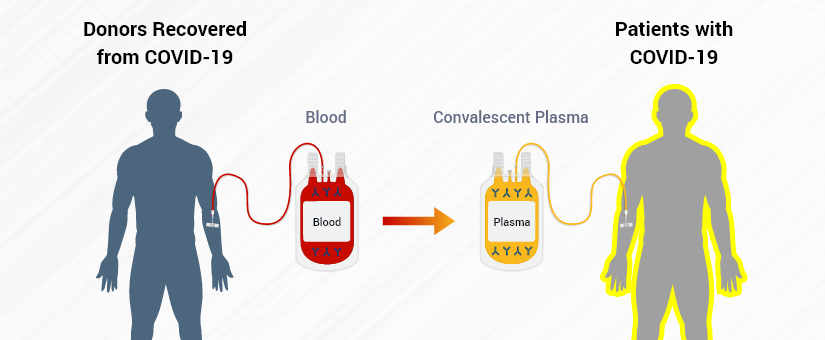
Convalescent plasma is defined as “blood plasma from people who have recovered from COVID-19 that may help others fight the disease.”
In other words, people who have had COVID-19 and recovered can participate in a blood plasma donation program such as those offered at our blood donation center in Seattle, Washington State.
This blood plasma contains COVID-19 antibodies, and when used to treat individuals with severe COVID-19, preferably within three days of diagnosis, or soon after their diagnosis, were less likely to die.
A Mayo Clinic study that enrolled over 35 000 patients, showed that patients who were given COVID-19 convalescent plasma within 3 days had a seven-day death rate of 8.7%. Juxtapositionally, patients who received this plasma after four or more days, had a mortality rate of 11.9%.
At this juncture, it is essential to note that this study did not include a placebo group, so the question of empirical evidence proving the impact of the plasma treatment is absent. However, the purpose of the study was to broaden access to plasma treatment. In this context, the study was successful.
What does the Emergency Use Authorization, or EUA, mean?
The fda.gov website defines the Emergency Use Authorizations as the allowance of the “unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when there are no adequate, approved, and available alternatives.”
As an aside, CBRN threat agents are Chemical, Biological, Radiological, and Nuclear threats.
In other words, the definition of a EUA allows the FDA to authorize the use of unapproved medical treatments and products to be used in an emergency to treat COVID-19. There is sufficient evidence, such as the Mayo Clinic study results, and because there are no currently approved COVID-19 treatments that the FDA acted as they did within the EUA guidelines.
Based on these definitions, the EUA for COVID-19 convalescent plasma to treat sufferers with severe symptoms is appropriate under the circumstances. In short, SARS-CoV-2 is a biological threat.
The importance of donating blood if you’ve recovered from COVID-19
At this point in the discussion, a brief explanation of what antibodies are and how they function to prevent a reoccurrence of COVID-19 is merited.
Livescience.com defines antibodies as “specialized, Y-shaped proteins that bind like a lock-and-key to the body’s foreign invaders,” such as the SARS-CoV-2 virus. Their primary function as part of the immune system’s search-and-destroy system is to find enemy cells or foreign bodies and mark them for destruction.
Therefore, people who have recovered from the illness (COVID-19) caused by the SARS-CoV-2 virus will have the antibodies that have successfully targeted this virus in their bloodstream. And over time, these individuals are protected from being reinfected by the virus.
There is a slight caveat here in that there are a few cases of people who have been reinfected by the virus. Because these cases seem to be the exception rather than the rule, this point is only mentioned for completeness.
Note: While other research laboratories might draw individual components of human blood and return the rest at the time of the draw, at we only draw whole blood and spin the different components out using a centrifuge.
Finding Solutions: Facilitating much-needed research into the SARS-CoV-2 virus
There is a second reason why people who have recovered from COVID-19 should sign up with our research laboratory as donors.
In summary, research institutions use biological products such as the COVID-19 convalescent plasma to provide answers to the many questions about the virus’s behavior.
Because it is a novel or new coronavirus, scientists and medical professionals know very little about its behavior. The good news is that, because the world is about six months into the global pandemic, answers have been forthcoming. These results provide vital insight into the virus’s behavior; thereby, helping medical professionals and national and federal governments prevent the spread of the virus, successfully treat sufferers, and prevent large fatalities.
Of course, the ultimate holy grail is the development of an effective vaccine against the virus.
Our laboratory uses donated human whole blood products to create in vitro COVID-19 convalescent plasma, specially designed to partner with the many research studies currently taking place, as well as future studies.
Conclusion
Contact our experts at Solomon Park Research Laboratories for assistance with your research study requirements.
Finally, call our donor center should you wish to sign up as a COVID-19 blood donor so that we can draw a blood donation for convalescent plasma.
Help us participate in the global fight against the COVID-19 pandemic.